Boron Neutron Capture Therapy (BNCT)
| What is Boron Neutron Capture Therapy? | |
| Dosimetry for BNCT | |
| A TEPC for BNCT | |
| Microdosimetric measurements |
An ideal therapy for cancer would be one whereby all tumour cells were selectively destroyed without damaging normal tissue. Most of the cancer cells should be destroyed for cure, either by treatment itself or with the help from the body’s immune system, otherwise the danger exists that the tumour may re-establish itself. Although today’s standard treatments - surgery, radiation therapy and chemotherapy - have successfully cured many kinds of cancers, there are still many treatments failures.
The BNCT (Boron Neutron Capture
Therapy) is a cancer treatment technique, which could be the best one
for those skin tumours (melanomas), which are nowadays resistant to ordinary
therapy. It makes use of thermal or epithermal neutrons to irradiate tumours
previously loaded with the stable isotope ![]() .
Thermal neutron absorption on the
.
Thermal neutron absorption on the ![]() nucleus gives rise to the production of two particles,
nucleus gives rise to the production of two particles, ![]() and
and ![]() , whose ranges
in tissue (~9 mm
and ~5 mm respectively) are as short as the
diameter of a cell nucleus and whose LET (Linear Energy Transfer) values
are largely high. Because of such short ranges, all the energy is released
inside the tumour cell, which is killed with high probability because
of the high LET values, while the neighbouring cells are not damaged.
, whose ranges
in tissue (~9 mm
and ~5 mm respectively) are as short as the
diameter of a cell nucleus and whose LET (Linear Energy Transfer) values
are largely high. Because of such short ranges, all the energy is released
inside the tumour cell, which is killed with high probability because
of the high LET values, while the neighbouring cells are not damaged.
The standard of practice for obtaining
the photon and neutron absorbed dose in BNCT is the dual dosimeter method,
in which one dosimeter is relatively insensitive to neutrons. The absorbed
dose due to the BNC reaction is not directly measured, but it is generally
calculated using activation foils to measure the thermal neutron flux
and the associated kerma coefficients for ![]() .
As a portion of the activation in the foil is induced by the epithermal
component of the beam, a second foil measurement is required to separate
the thermal flux from the epithermal flux. A total of four measurements
at each point are therefore required to obtain the photon, neutron and
BNC absorbed dose components. Large uncertainties are ascribed to these
techniques when used for BNCT dosimetry.
.
As a portion of the activation in the foil is induced by the epithermal
component of the beam, a second foil measurement is required to separate
the thermal flux from the epithermal flux. A total of four measurements
at each point are therefore required to obtain the photon, neutron and
BNC absorbed dose components. Large uncertainties are ascribed to these
techniques when used for BNCT dosimetry.
Dosimetry using tissue equivalent proportional counters (TEPCs) allows the direct measurement of the BNC dose, yields smaller uncertainties in the neutron absorbed dose and requires only two measurements at each point to provide all three dose components. Moreover, lineal energy spectra measured with TEPCs can be used to predict the RBE through the use of biological weighting functions.
The general formalism for presentation of microdosimetric spectra collected with TEPCs is the lineal energy spectrum. Lineal energy, y, is defined as the energy imparted in a volume divided by the mean chord length of the volume:
![]()
For a TEPC with right cylindrical collecting volume, the mean chord length is equal to 2/3 the diameter of the cylinder, d. This yields the following expression for the calculation of absorbed dose from a lineal energy spectrum:
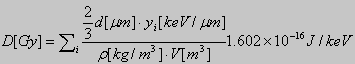
where r is the density of the gas, V
the volume of the cavity and ![]() the
lineal energy deposited by the j
the
lineal energy deposited by the j![]() charged particle crossing the counter volume.
charged particle crossing the counter volume.
The so called “dual counter microdosimetric
technique” utilize two detectors of different wall materials, A-150
tissue-equivalent plastic and boron-loaded A-150 plastic. If the two detectors
are otherwise identical, the lineal energy spectrum resulting from the
BNC reaction may be obtained from the difference in spectra measured using
these two TEPCs.
We have constructed a TEPC, which could
monitor the fluctuation of the absorbed energy, namely the microdosimetric
spectra, both in a 1 µm size site and in a 50 nm size site (at density
of 1g/![]() ). These
sites have the dimensions of a chromosome and of a mitochondrion respectively.
In this way it is possible to assess the effects of different subcellular
localization of boron compounds.
). These
sites have the dimensions of a chromosome and of a mitochondrion respectively.
In this way it is possible to assess the effects of different subcellular
localization of boron compounds.
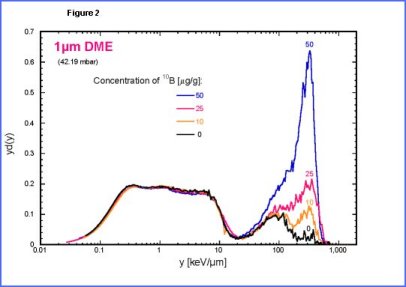
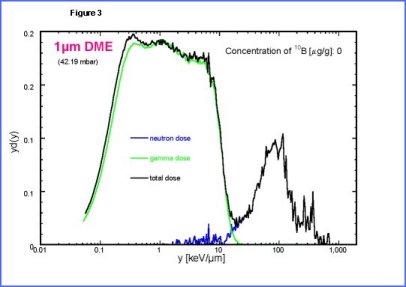
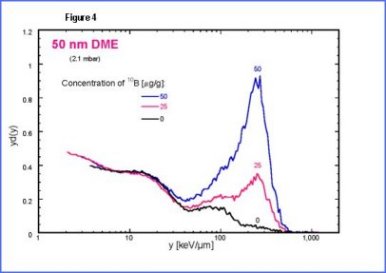
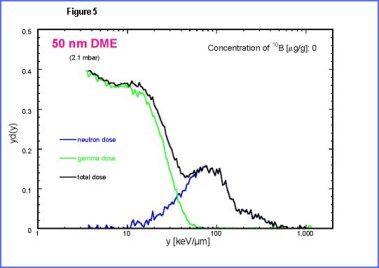
| The
second peak is due to low energy protons set in motions by neutrons.
The third peak is mainly due to |
The Legnaro National
Laboratory (LNL) of the INFN is studying the construction of a specialised
facility (SPES: Study and Production of Exotic Species)
for Radioactive Ion Beams (RIB) originated by fission fragments produced
by secondary neutrons. This facility will allow also having intense neutron
beams. It will allow for carrying out significant experiments and activities
in both fundamental and applied nuclear physics (medicine, biology and
In view of this application we will continue with our TEPC the study
of the microdosimetric properties of neutron beams suitable for BNCT,
both at the LNL and also at the TAPIRO reactor of ENEA laboratory at Casaccia.
However, future therapeutic beams will be very intense. A typical therapeutic
beam could have intensity of the order of ![]() .
Under these conditions pile-up effects, which spoil microdosimetric spectra,
are not avoidable with ordinary TEPCs of about 1 cm of diameterpeak. With such intense beams
the monitoring can be performed only with mini-TEPC of 1
.
Under these conditions pile-up effects, which spoil microdosimetric spectra,
are not avoidable with ordinary TEPCs of about 1 cm of diameterpeak. With such intense beams
the monitoring can be performed only with mini-TEPC of 1![]() of
cavity, which are able to work properly up to
of
cavity, which are able to work properly up to ![]() of counting rate. We have already developed mini-TEPCs for adrontherapy
. For BNCT we will develop mini-TEPCs with
of counting rate. We have already developed mini-TEPCs for adrontherapy
. For BNCT we will develop mini-TEPCs with ![]() added to the tissue-equivalent plastic of the cathode wall. These TEPCs
will be small enough to provide phantom dosimetry with excellent spatial
resolution at clinical beam intensities.
added to the tissue-equivalent plastic of the cathode wall. These TEPCs
will be small enough to provide phantom dosimetry with excellent spatial
resolution at clinical beam intensities.
- V. Cesari, L. De Nardo, P. Colautti, G. Tornielli, M. Muller-Veggian,
G. Donà.
First microdosimetric measurements down to 25 nm. LNL Annual Report 2000 LNL-INFN (REP): 82-83 - A. Alkaa, W. Y. Baek, M. C. Bordage, A. Breskin, R. Chechik, P. Colautti, V. Conte, L. De Nardo, B. Grosswendt, J. Kula, G. Magrin, S. Marjanska, I. C. McDougall, S. Pszona, P. Ségur, S. Shchemelinin, J. Tamboul, G. Tornielli, D. E. Watt - LNL-INFN(REP)-161/00 (2000)
- S. Agosteo, L. Casoli, V. Cesari, P. Colautti, N.Colonna, V. Conte,
G. Curzio, L. De Nardo, F. d'Errico, G. Donà, C. Fabris, G. Fortuna,
G. Gambarini, M. Geronazzo, F. Giuntini, G. Jori, M. Lollo, G. Roncucci,
G. Sotti, L. Tecchio, R. Tinti, G. Tornielli.
Advances in the INFN-Legnaro BNCT project for skin melanoma. Proceedings of the International Physical and Clinical workshop on BNCT. Held in Candiolo (Torino) on the 17th of Febbruary 2001 - V. Cesari ,L.De Nardo , P. Colautti ,V.Conte ,G.Tornielli ,J.Esposito, M. Müller-Veggian,G.Donà. First microdosimetric measurements with a 10 B-loaded TEPC. LNL Annual Report 2001 LNL-INFN (REP):110-111
- V. Cesari, P.Colautti, G. Magrin, L.De Nardo,W. Y. Baek, B. Grosswendt,
A.Alkaa, C. Khamphan, P.Ségur and G.Tornielli. Nanodosimetric
Measurements with an Avalanche Confinement TEPC. Radiat. Prot. Dosim.
99: 337-341 (2002)